![Imidafenacin.png]()
Imidafenacin
イミダフェナシン
Cas 170105-16-5
C20H21N3O, 319.408
APPROVED JAPAN 2015-07-29
|
4-(2-methyl-1H-imidazol-1-yl)-2,2-diphenylbutanamide
|
4-(2-methylimidazol-1-yl)-2,2-di(phenyl)butyramide
D06273
KRP-197
KRP-197;ONO-8025
ONO-8025
UNII:XJR8Y07LJO
Company:Kyorin (Originator), Ono (Originator)
4-(2-methyl-1-imidazolyl)- 2,2-diphenylbutyramide as a colorless needle: mp 189.0±190.0 C (from ethyl acetate:ethanol);
High MS (EI+) m/z calcd for C20H21N3O 319.1685, found 319.1671;
1 H NMR (400 MHz, CDCl3) d 2.23 (3H, s), 2.69±2.74 (2H, m), 3.77±3.82 (2H, m), 5.33 (1H, s), 5.49 (1H, s), 6.73 (1H, s), 6.85 (1H, s), 7.31±7.42 (10H, m).
イミダフェナシン
Imidafenacin
![]()
C
20H
21N
3O : 319.4
[170105-16-5]
Imidafenacin (INN) is a urinary antispasmodic of the anticholinergic class. It’s molecular weight is 319.40 g/mol
| Imidafenacin (INN) is a urinary antispasmodic of the anticholinergic class. |
Kyorin and Ono have developed and launched imidafenacin, an oral M1 and M3 muscarinic receptor antagonist. Family members of the product case, WO9515951, expire in the US in 2019
Imidafenacin was approved by Pharmaceuticals Medical Devices Agency of Japan (PMDA) on Apr 18, 2007. It was marketed as Uritos® by Kyorin, and marketed as Staybla® by Ono.
Imidafenacin is a potent M1 and M3-subtype antagonist indicated for the treatment of urinary urgency, frequent urination and urgency urinary incontinence due to overactive bladder.
Uritos® is available as tablet for oral use, containing 0.1 mg of free Imidafenacin. The recommended dose is 0.1 mg twice daily, and it can be increased to 0.2 mg twice daily, if the efficacy was not enough.
Uritos® / Staybla®
![Image result for Uritos®]()
![Image result for Staybla®]()
MOA:Muscarinic acetylcholine receptor antagonist
Indication:Urinary incontinence; Urinary urgency and frequency
![ChemSpider 2D Image | Imidafenacin | C20H21N3O]()
![Image result for KYORIN]()
PAPER
WO-2016142173
Imidafenacin, the compound of formula (I), is an antimuscarinic agent marketed in Japan under the brand name Uritos® used to treat overactive bladder, a disease defined by the presence of urinary urgency, usually accompanied by frequency and nocturia, with or without urge incontinence. Overactive bladder dysfunction has a considerable impact on patient quality of life, although it does not affect survival.
![]()
(I)
Synthesis of 4-(2-methyl-1 -imidazolyl)-2,2-diphenylbutanamide is first disclosed in Japanese patent JP3294961 B2 as shown in Scheme 1 . 4-bromo-2,2-diphenylbutanenitrile (II) is reacted with three equivalents of 2-methylimidazol, in dimethylformamide and in the presence of triethylamine as a base, to afford 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile, compound of formula (III), which is purified by column chromatography and, further, converted into its hydrochloride salt and recrystallized. Then, compound (III) is hydrolyzed with an excess of 70% sulfuric acid at 140-150 °C, followed by basification and recrystallization to provide imidafenacin (I), in an overall yield of only 25% (as calculated by data provided in docume
![]()
(III) (I)
Scheme 1
This route of document JP3294961 B2 implies several drawbacks. Firstly, purification of intermediate (III) is carried out by means of chromatographic methods, which are generally expensive, environmentally unfriendly and time consuming. Secondly, the hydrolysis of the nitrile group is carried out under strong acidic conditions and high temperature not convenient for industrial application.
Japanese document JP2003-201281 discloses a process for preparing imidafenacin as shown in Scheme 2. 4-bromo-2,2-diphenylbutanenitrile (II) is reacted, with five equivalents of 2-methylimidazol, which acts also as a base, in dimethylsufoxide to provide intermediate (III), which after an isolation step is further reacted with phosphoric acid in ethanol to provide the phosphate salt of 4-(2-methyl-1 H-imidazol-1-yl)-2,2-diphenylbutanenitrile. Hydrolysis with potassium hydroxide, followed by purification with a synthetic adsorbent provides imidafenacin (I) in a moderate overall yield
![]()
(II) (I)
Scheme 2
The use of a synthetic adsorbent is associated with problems with operativities and purification efficiencies from the viewpoint of industrial production, therefore, the process disclosed in document JP2003-201281 is not suitable for industrial application.
EP1845091 A1 discloses a process for preparing imidafenacin, according to previous document JP2003-201281 , however the purification step is carried out by either preparing the hydrochloride or the phosphate salt of imidafenacin followed by neutralization as shown in Scheme 3. Purified imidafenacin is provided in low yield, overall yield of about 31 % (as calculated by data provided in document EP1845091 A1 ). This process has several disadvantages. Firstly, EP1845091 A1 states that the penultimate intermediate, the 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile phosphate is hygroscopic, which implies handling problems. Secondly, the additional steps carried out for purification increases the cost of the final imidafenacin process and the pharmaceutical compositions containing it, which already resulted in expensive medications.
![]()
(II) (I)
HCI or
H3PO4
![]()
purified (I) HCI or Ή3ΡΟ4
Scheme 3
The intermediate phosphate salt of 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile obtained and used in prior art processes is a solid form having needle-shaped crystals, which are difficult to filtrate. Moreover, said needle-shaped crystals are very hygroscopic and unstable and transform over time to other solid forms. In addition, the water absorbed by this solid form described in the prior art may react with the intermediate to generate further impurities.
Therefore, there is still a need to develop an improved industrially feasible process for the manufacture of imidafenacin in good purity and good yield, involving the use of stable intermediates having also improved handling characteristics.
Example 1 :
Preparation of 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile phosphate in solid Form I
4-bromo-2,2-diphenylbutanenitrile (II, 1.000 Kg, 3.33 mol) and 2-methylimidazol (1 .368 Kg, 16.66 mol) were heated in DMSO (0.8 L) at 100-105 °C for 7 hours. The solution was then cooled to 20-25 °C and toluene (2 L) and water (4 L) were added and stirred for 30 minutes. After phase separation, the aqueous layer was extracted with toluene (1 L). Organic layers were combined and washed twice with water (2 x 1 L). Distillation of toluene provided 4-(2-methyl-1 H-imidazol-1-yl)-2,2-diphenylbutanenitrile as a brown oil (0.915 Kg), which was, then, dissolved in dry acetone (3 L) and water (0.1 L), heated to 40-45°C and seeded with 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile phosphate. A solution of orthophosphoric acid (0.391 Kg, 3.39 mol) in acetone (2 L) was then added dropwise, maintaining temperature at 40-45 °C. Once the addition was finished, the reaction mixture was maintained 1 hour at 40-45 °C, cooled to 20-25 °C and stirred for 1 hour. The solid was filtered, washed with acetone (1 L), suspended in 2-propanol (10 L), heated at 80 °C and 2 L of solvent were distilled. The obtained suspension was then seeded with 4-(2-methyl-1 H-imidazol-1 -yl)-2,2-diphenylbutanenitrile phosphate solid Form I and maintained at 80 °C for 5 hours. The suspension was cooled down to 20-25°C, filtered off, washed with 2-propanol (1 L) and, finally, dried (45 °C, 0.5 torr, 12 hours).
Yield: 0.967 Kg (73%)
HPLC: 99.5 %
KF: 0.2 %
Optical microscopy: plate-shaped crystal habit as substantially in accordance to Figure 2.
PSD: D90 of 105 m
PXRD: Crystalline solid form as substantially in accordance to Figure 3.
DSC (10 °C/min): Endothermic peak with onset at 177 °C (-1 18 J/g), as substantially in accordance to Figure 4.
TGA (10 °C/min): Decomposition starting at 180 °C.
DVS: No significant weight gain up to 90% of relative humidity. At this humidity, a total increase of only 0.45% in weight was observed.
SCXRD: Crystal structure substantially in accordance to Figure 5. There are not water or solvent molecules in the crystal structure.
PATENT
https://www.google.com/patents/CN103351344A?cl=en
Overactive Bladder (symptomatic overactive bladder, 0AB) is a common chronic lower urinary tract dysfunction. Its incidence, United States and Europe over 75 year-old male incidence up to 42%, slightly lower incidence of women 31%; the incidence of domestic in Beijing 50 years of age for men was 16.4% for women over the age of 18 mixed The overall incidence of urinary incontinence and urge incontinence was 40.4 percent, seriously affecting the physical and mental health of the patient, reduced quality of life. Common antimuscarinic drugs in vivo and in vivo M receptor in some or all of binding with different affinities to improve the symptoms of OAB, but will also cause many side effects, such as dry mouth, constipation, cognitive impairment , tachycardia, blurred vision and so on. Imidafenacin have diphenylbutanoic amide structure, is a new high anticholinergic drugs, which selectively acts on the M3 and Ml receptors, blocking the contraction of the detrusor choline, so detrusor relaxation, reduce side effects of drugs. Meanwhile imidafenacin inhibit smooth muscle of the bladder and inhibiting acetylcholine free dual role, and selectivity for the bladder stronger than the salivary glands.
imidafenacin is a new diphenylbutanoic amides from Japan Ono Pharmaceutical Co., Ltd. jointly developed with Kyorin Pharmaceutical anticholinergics, structure (I) as follows:
The goods listed in June 2007 in Japan under the trade name: STAYBLA, chemical name: 4- (2-methyl-1-imidazolyl) _2,2- diphenylbutyric amide.
At present the preparation imidafenacin few reports, can be summed up as the following ways:
China Patent CN10699098 reported to bromoethyl diphenyl acetonitrile and 2-methylimidazole as a raw material, at 150 ° C condition, after the reaction DMF / triethylamine system, sulfuric acid hydrolysis reuse imidafenacin. The reaction equation is as follows:
BACKGROUND OF THE INVENTION This two-step method was 24% overall yield is too low, and the second step of the reaction is difficult to control. And the reaction product was purified by column chromatography required to obtain a purified product, is not conducive to industrial production.
Chinese patent CN101362721A referred to as the hydrolysis conditions for the preparation of sulfuric acid and organic acid mixed use imidafenacin yield have mentioned the smell.
Although this method increases the yield, but still more by-product of the reaction, the product is not easy purification.
Japanese Patent No. JP2005 / 023216 proposes hydrolysis under alkaline environment, and the use of products and solutions of salts hydrochloride salt and then purified product.
This method improves the yield of the second step of the hydrolysis reaction and simplified purification methods. But the need to use this method to purify salt activated carbon, and filtration devices require more stringent; and a need to be re-crystallized salt solution salt after the operation, a total of four steps of unit operations. Process more cumbersome and more stringent requirements for equipment, it is not conducive to industrial scale production. In addition, the product is dried for a long time, still remaining after solvent treatment product obtained, the purity of the product is still low.
DETAILED DESCRIPTION
The following typical examples are intended to illustrate the present invention, simple replacement of skill in the art of the present invention or improvement made in all part of the present invention within the protection of technical solutions.
Example 1
4- (2-methyl-1-imidazolyl) -2,2-diphenyl butanamide hydrobromide. The 16.5 g (52 mmol) 4- (2- methyl-1-imidazolyl) -2,2-diphenyl butyramide crude into 100 mL of isopropanol, stirring was added 8.0 mL hydrobromic acid and isopropyl alcohol mixed solution (volume ratio of 1: 1), the solid gradually dissolved, was nearly colorless and transparent liquid. After maintaining the reaction mixture was stirred for half an hour, the reaction mixture was added to 100 mL of ethyl acetate, stirred for I hour at room temperature, solid precipitated. Filtration, and the cake was rinsed with an appropriate amount of ethyl acetate. The solid was collected, 40 ° C drying oven and dried to constant weight to give 19.5 g white 4- (2-methyl-1-imidazolyl) -2,2-diphenyl butyramide hydrobromide, yield 98.9%. ?] \ 1 .228.4-229.00C0MS (m / z): 320 [M + 1] +. 1H-NMR (DMS0-1 / 6, 400 MHz) δ: 2.25 (3H, s), 2.73-2.74 (2H, m), 3.68-3.91 (2H, m), 6.81 (1H, s), 7.28-7.35 (I OH, m), 7.39 (1H, s), 7.49 (1H, d, /=2.4 Hz), 7.55 (1H, d, J = 2.2 Hz), 14.39 (1¾ br s).
Example 2
4- (2-methyl-1-imidazolyl) -2,2-diphenyl butyramide. -2,2-Diphenyl butyric acid amide acetate was dissolved in 900 mL of water to 19.5 g (0.051mmol) obtained in Example 1 4- (2-methyl-1-imidazolyl) embodiment. Extracted with 900mL diethyl ether solution, collecting the inorganic layer. Was added to an aqueous solution of 200 mL of ethanol, was added to the system with stirring in an aqueous solution of KOH 2mol / L, there is a solid precipitated. The reaction was stirred I h after filtration. Cake was washed with 40% ethanol solution rinse, rinsed with water several times. Collect the cake, put 40 ° C drying oven dried to constant weight to give 14.8 g white 4- (2-methyl-1-imidazolyl) -2,2-diphenyl methylbutanamide, yield 91.0% (total yield 90% two steps). Μ.p.192.3-193.00C (CN101076521A 191-193O). MS (m / z): 320 [M + l] +. 1H-NMR (DMSO-J6, 400MHz) δ: 2.11 (3Η, s), 2.69-2.73 (2H, m), 3.61-3.65 (2H, m), 6.75 (1H, d, J = L OMHz), 7.01 (1H, br s), 7.04 (1H, d, J = L 0 MHz), 7.34-7.49 (11H, m).
Example 3
4- (2-methyl-1-imidazolyl) -2,2-diphenyl butyramide. The 14.5 g (0.045mmol) obtained in Example 4- (2-methyl-1-imidazolyl) -2,2-diphenyl butanamide 2 was added 116 mL of ethyl acetate was slowly heated to reflux reflux for 30 min, cooled to room temperature for crystallization 5 h. Suction filtered, the filter cake was rinsed with a small amount of ethanol, collected cake was put 40 ° C drying oven and dried to constant weight to give 13.4 g white 4- (2-methyl-1-imidazolyl) -2,2- diphenyl methylbutanamide refined products, yield 92.4% (three-step total yield 83.1%). Mp192.5-193 (TC (CN101076521A 191_193 ° C) .MS (m / z):.. 320 [M + 1] + 1H-NMR (DMSO-J6, 400 MHz) δ
2.11 (3H, 7.01 (1H,
s), 2.69-2.73 (2H, br s), 7.04 (1H, d,
m), 3.61-3.65 (2H, m), 6.75 (1H, J = L 0 MHz), 7.34-7.49 (11H, m).
![Image result for Imidafenacin]()
PATENT
CN103772286A.
imidafenacin (Imidafenacin) is a new diphenylbutanoic amides from Japan Ono Pharmaceutical Co., Ltd. jointly developed with Kyorin Pharmaceutical anticholinergic drugs, bladder is highly selective for the treatment of overactive bladder, in 2007 in June in Japan. Its chemical name is 4- (2-methyl -1H- imidazol-1-yl) -2,2-diphenyl butyramide chemical structure shown by the following formula I:
Reported in U.S. Patent No. US5932607 imidafenacin preparation method, the method is based on 4-bromo-2 ‘2 ~ phenyl butyronitrile, 2-methylimidazole, triethylamine as raw materials, with DMF as a solvent at 150 ° C reaction 30h, to give the intermediate 4- (2-methyl-imidazol-1-yl) -2,2-diphenyl-butyronitrile, 77% yield, then body 140 ~ 150 ° C with 70% sulfuric acid The resulting intermediate hydrolyzed to the amide, after completion of the reaction required excess soda and sulfuric acid, the reaction is as follows:
Which preclude the use of the dilute sulfuric acid hydrolysis, although succeeded in getting the product, but the yield is very low, only 32%, greatly increasing the production cost, mainly due to 70% sulfuric acid, the reaction is difficult to control amide phase, the product will continue to acid hydrolysis byproducts, resulting in decreased yield.
European Patent No. EP1845091 reports imidafenacin Another preparation method, the method using potassium hydroxide and isopropyl alcohol 4- (2-methyl-imidazol-1-yl) diphenyl _2,2- Hydrolysis of nitrile to amide phosphates, and the crude product was converted to the hydrochloride or phosphate, and recrystallized to remove impurities and then basified imidafenacin obtained, which reaction is as follows:
This method uses a lot of bases, product purification is too much trouble, and the total yield of 45%.
Chinese Patent Publication No. CN102746235 also disclosed imidafenacin preparation method of 4- (2-methyl-1-yl) -2,2-diphenyl phosphate or nitrile salt in methanol / ethanol, dimethyl sulfoxide, and the presence of a base, with hydrogen peroxide in 40 ~ 60 ° C under through improved Radziszewski the target compound, the reaction is as follows:
The method used in the hydrogen peroxide solution, but a solution of hydrogen peroxide has strong oxidizing, and has a certain corrosive, inhalation of the vapor or mist respiratory irritation strong, direct eye contact with the liquid may cause irreversible damage and even blindness, security It is not high on the human body and environmentally unfriendly. Alkaline environment, easily decomposed hydrogen peroxide, as the temperature increases, the decomposition reaction increased, and therefore reaction requires a large excess of hydrogen peroxide solution.
The method comprises the steps of: (1) 4-Bromo-2,2-diphenyl-butyronitrile is hydrolyzed to the amide under basic conditions; (2) The obtained 4-bromo-2,2-diphenylbutyric amide is reacted with 2-methylimidazole to give the desired product.
Example 1
2L reaction flask was added 400mL of dry tetrahydrofuran, under a nitrogen atmosphere was added 60% sodium hydride (82.8g, 2.06mol), stirred to obtain a gray turbid solution A. With 400mL dry tetrahydrofuran was sufficiently dissolved diphenyl acetonitrile (200g, 1.04mol), I, 2- dibromoethane (204.2g, 1.08mol), to give a colorless clear liquid B; 5 ~ 15 ° C, a solution of turbid solution B dropwise to solution A, 10 ~ 15 ° C the reaction was incubated 6h, TLC until the reaction was complete, to the reaction system a small amount of water was added dropwise until no bubbles. After addition of 800mL water, 400mL ethyl acetate and stirred, liquid separation, the organic layer was washed with water, saturated sodium chloride solution, respectively, and the organic layer was dried over anhydrous sodium sulfate, suction filtered, concentrated under reduced pressure to give a yellow liquid 310g.
[0018] The resulting yellow liquid with 800mL 90% ethanol and stirred to dissolve at 40 ° C, then cooling and crystallization, filtration, 45 ° C and concentrated under reduced pressure to give a white solid 232.8g, 75% yield.
Preparation of bromo-2,2-diphenyl 4_ butanamide: [0019] Example 2
3L reaction flask was added 4-bromo-2,2-diphenyl-butyronitrile (15 (^, 0.511101), 7501 ^ 6mol / L KOH solution, 750mL dimethylsulfoxide and heated to 100 ~ 120 ° C under stirring The reaction, the reaction lh, until the reaction was complete by TLC after cooling to 40 V, add 2000mL water, 2000mL of methylene chloride was stirred, liquid separation, the organic layer was washed with water, washed with saturated sodium bicarbonate and sodium chloride solution, separated, dried over anhydrous The organic layer was dried over sodium sulphate, filtration, concentrated under reduced pressure to give brown oily liquid 161.92g, 96% yield.
Preparation of bromo-2,2-diphenyl 4_ butanamide: [0020] Example 3
3L reaction flask was added 4-bromo-2,2-diphenyl-butyronitrile (150g, 0.5mol), 666mL 6mol / L NaOH solution, 750mL dimethylsulfoxide, the reaction mixture was stirred and heated to 100 ~ 120 ° C under The reaction lh, until the reaction was complete by TLC after cooling to 40 ° C, add water 2000mL, 2000mL of methylene chloride was stirred, liquid separation, the organic layer was washed with water, washed with saturated sodium bicarbonate and sodium chloride solution, separated, dried over anhydrous sulfate sodium organic layer was dried, filtration, concentrated under reduced pressure to give brown oily liquid 146.73g, 87% yield.
Preparation of bromo-2,2-diphenyl 4_ butanamide: [0021] Example 4
The reaction was stirred 3L reaction flask was added 4-bromo-2,2-diphenyl-butyronitrile (15 (^, 0.511101), 8331 ^ 36% Na2CO3 solution, 750mL dimethylsulfoxide and heated to 100 ~ 120 ° C under The reaction lh, until the reaction was complete by TLC after cooling to 40 ° C, add water 2000mL, 2000mL of methylene chloride was stirred, liquid separation, the organic layer was washed with water, washed with saturated sodium bicarbonate and sodium chloride solution, separated, dried over anhydrous The organic layer was dried over sodium sulphate, filtration, concentrated under reduced pressure to give brown oily liquid 153.48g, yield 91%.
`[0022] Example 5: 4- (2-methyl-imidazol _1_ -1H- yl) butyramide _2,2_ diphenyl (imidafenacin) Preparation 5L reaction flask was added 4-bromo-2 2-diphenyl butyric amide (160g, L 5mol), 2- methyl imidazole (123g,
1.5mol), triethylamine (50.6g, 0.5mol), potassium iodide (5g, 0.03mol), fully dissolved with 1000mL DMF solution was heated to 120 ° C at a reaction 5h, until completion of the reaction by TLC, heating was stopped, to be After cooling, water was added 3000mL system stirred 0.5h, filtration, washed with water until the filtrate is neutral, concentrated under reduced pressure and dried to give a brown solid 146.14g, a yield of 91%.
[0023] Example 6: 4- (2-methyl-imidazol _1_ -1H- yl) butyramide _2,2_ diphenyl (imidafenacin) Preparation 5L reaction flask was added 4-bromo-2, 2- diphenyl butyramide (160g, 0.5mol), 2- methyl imidazole (82.1g,
1.011101), triethylamine (50.68,0.5mol), potassium iodide (5g, 0.03mol), fully dissolved with 1000mL DMF solution was heated to 120 ° C at a reaction 5h, until completion of the reaction by TLC, heating was stopped, the system was cooled until After adding 3000mL water, stirring 0.5h, filtration, washed with water until the filtrate is neutral, concentrated under reduced pressure and dried to give a brown solid 120.45g, 80% yield.
[0024] Example 7: 4- (2-methyl-imidazol _1_ -1H- yl) butyramide _2,2_ diphenyl (imidafenacin) Preparation 5L reaction flask was added 4-bromo-2, 2- diphenyl butyramide (160g, 0.5mol), 2_ methylimidazole (164.2g,
2.011101), triethylamine (50.68,0.5mol), potassium iodide (5g, 0.03mol), fully dissolved with 1000mL DMF solution was heated to 120 ° C at a reaction 5h, until completion of the reaction by TLC, heating was stopped, the system was cooled until After adding water, stirring 3000mL
0.5h, suction filtered, washed with water until the filtrate was neutral, and concentrated under reduced pressure, and dried to give a brown solid 141.33g, yield 88%.
[0025] Example 8: 4- (2-methyl imidazole -1H- _1_ group) _2,2_ diphenylbutanoic amide (imidafenacin) refining up to 80g microphone said that new crude added 300mL of absolute ethanol, the system was warmed to reflux, refluxed
0.5h, after cooling the ethanol was distilled off to IOOmL about 500mL of ethyl acetate was added to precipitate a white solid, a small amount of ethyl acetate and wash the filter cake, 45 ° C and dried in vacuo to give 74.6g of white crystals, yield 93%.
![]()
CLIP
| EP 0733621; US 5932607; US 6103747; WO 9515951 |
![]()
![Image result for Imidafenacin]()
Alkylation of diphenylacetonitrile (I) with dibromoethane provided bromide (II). This was condensed with 2-methylimidazole (III) in the presence of Et3N in DMF to afford the substituted imidazole (IV). Finally, hydrolysis of the cyano group of (IV) with 70% sulfuric acid produced the target amide.
![]()
Treatment of acetonitrile derivative (I) with dibromoethane (II) in toluene in the presence of NaNH2 affords bromo compound (III), which is then condensed with imidazole derivative (IV) by means of Et3N in DMF to provide compound (V). Hydrolysis of the cyano group of (V) with aqueous H2SO4 yields amide derivative (VI), which is finally subjected to alkyl quaternization by reaction with bromobenzyl bromide (VI) in acetone to furnish the desired product.
Paper
Bioorganic & Medicinal Chemistry Letters 9 (1999) 3003-3008
![]()
![]()
PAPER
Bioorganic & Medicinal Chemistry 7 (1999) 1151±1161
![]()
4-(2-methyl-1-imidazolyl)- 2,2-diphenylbutyramide (2.02 g, 24%) as a colorless needle:
mp 189.0±190.0 C (from ethyl acetate:ethanol);
High MS (EI+) m/z calcd for C20H21N3O 319.1685, found 319.1671;
1 H NMR (400 MHz, CDCl3) d 2.23 (3H, s), 2.69±2.74 (2H, m), 3.77±3.82 (2H, m), 5.33 (1H, s), 5.49 (1H, s), 6.73 (1H, s), 6.85 (1H, s), 7.31±7.42 (10H, m).
PATENT
CN103880751A.
imidafenacin chemical name 4- (2-methyl–1H–1-yl) -2,2-diphenyl methylbutanamide (I).
In Patent JP93-341467, JP94-319355 and literature Bioorganic & Medicinal ChemistryLetters, 1999, vol.9,3003 – 3008 reported in the chemical synthesis routes to diphenyl acetonitrile (4) as the starting material,
Condensation and hydrolysis reaction step to give imidafenacin (1).
The new method is simple, mild reaction conditions, easy to control, good high yield and purity of the product, do not pollute the environment, suitable for industrial production.
[0012] The first method from 2-methylimidazole and I, 2- dibromoethane under phase transfer catalyst is tetrabutylammonium bromide (TBAB) and inorganic base catalyzed generate 1- (2-bromoethyl) – methyl -1H- imidazole (5), and diphenyl acetonitrile (4) a phase transfer catalyst and an inorganic base catalyzed condensation of 4- (2-methyl–1H- imidazol-1-yl) -2,2 – diphenylbutyronitrile hydrochloride (2), and then hydrolyzed to imidafenacin (I)
FIG. 1 imidafenacin IH-NMR spectrum
![]()
FIG. 2 imidafenacin 13C-NMR spectra
![]()
Examples I
1- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), tetrabutylammonium bromide (TBAB) (0.5g) and K2C03 (3.6g), K0H ( 4.6g) were added sequentially 100mL three-necked flask and stirred and heated to 50 ° C reaction 7h. Cooling to room temperature, the reaction solution was filtered, and the filtrate was washed with saturated aqueous sodium bicarbonate, dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was stirred resolved crystal dissolved, to give the product 5.lg, yield 88.5%, mp.79_80 ° C.
Preparation of 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
The diphenyl acetonitrile (5.8g, 30mmol) and 50% aqueous KOH (15ml), dimethyl sulfoxide (DMSO) (100ml), tetrabutylammonium bromide (TBAB) (0.9g) in toluene 50ml was added to the reaction flask and stirred for 0.5h in the 40 ° C. 1- (2-bromoethyl) -2-methyl -1H- imidazole (4) (5.lg, 27mmol), was heated to 20 ° C, the reaction was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL water, extracted three times with ethyl acetate 240ml water phase. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Analytical crystal solution with hydrogen chloride ether solution, filtered crystals with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,2-diphenylbutyric carbonitrile hydrochloride (2) of a white solid 7.lg, yield 77.8%, mp: 156.5-158 ° C. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.35-7.42 (IOH, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 ( 2H, m), 2.25 (3H, s).
The preparation imidafenacin (I),
4- (2-methyl-1-imidazolyl) -2,2_ diphenyl butyronitrile hydrochloride (2) (8.78g, 26mmol) in 70% concentrated sulfuric acid (25ml) was added to the reaction bottle, the reaction was stirred at 90 ° C, the end of the reaction was monitored by TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 7.0g, yield 84.5%, mp: 188.0-190 (. TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2-),
3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H)
, 6.828 (s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H).
[0030] 13C-NMR (CDC13,400MHz) δ: 12.17 (-CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
Example 2
[0032] 1- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
[0033] The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), tetrabutylammonium chloride (0.43g) and Na2CO3 (2.8g), NaOH (3.3g) followed by adding 100mL three-necked flask, stirred and heated to 40 ° C reaction 5h.Cooling to room temperature, the reaction solution was filtered, and the filtrate was washed with saturated aqueous sodium bicarbonate, dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was dissolved with stirring parsing crystal give the product 4.9g, yield 85.1%, mp.79-80 ° C.
Preparation of [0034] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
[0035] A two phenylethyl chest (5.8g, 30mmol) and 50% aqueous NaOH (15ml), dimethylethylene Bitterness (DMSO) (100ml), tetrabutylammonium chloride (0.8g) was added to a toluene 50ml The reaction flask, stirred 0.5h in the 40 ° C. Join
1- (2-bromoethyl) -2-methyl -1H- imidazole (4) (5.lg, 27mmol), was heated to 60 ° C, the reaction was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL of water and extracted three times with ethyl acetate 240ml water phase. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Solution of hydrogen chloride in ether solution with analytical crystal, crystals were filtered with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,
2-phenyl-butyronitrile hydrochloride (2) as a white solid 7.0g, yield 76.8%, mp: 156.5-158 ° C. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.35-7.42 (IOH, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 ( 2H, m), 2.25 (3H, s).
Preparation imidafenacin (I),
[0037] 4- (2-methyl-1-imidazolyl) -2,2_ diphenyl butyronitrile hydrochloride (2) (8.78g, 26mmol) in 70% concentrated sulfuric acid (25ml) was added to the reaction bottle, the reaction was stirred at 110 ° C, the end of the reaction was monitored by TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 7.2g, yield 86.8%, mp: 188.0-190 (. TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2-),
3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H), 6.828 ( s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H).
13C-NMR (CDC13,400MHz) δ: 12.17 (-CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
Example 3
[0041] 1- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
[0042] The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), benzyltriethylammonium chloride (TEBA) (0.35g) and Na2CO3 (2.8g), Na0H (3.3g) were added sequentially 100mL three-necked flask, stirred and heated to 45 ° C reaction 4h. Cooling to room temperature, the reaction solution was filtered, washed with a saturated aqueous sodium bicarbonate paint filtrate was dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was dissolved with stirring parsing crystal give the product 5.0g, yield 86.8%, mp.79-80. . .
Preparation of [0043] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
The diphenyl acetonitrile (5.8g, 30mmol) and 50% aqueous KOH (15ml), dimethyl sulfoxide (DMSO) (100ml), benzyltriethylammonium chloride (TEBA) (0.66g) 50ml Toluene was added to the reaction flask and stirred at 40 ° C under
0.5h0 was added 1- (2-bromoethyl) -2-methyl -1H- imidazole (4) (5.lg, 27mmol), was heated to 60 ° C, the reaction was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL of water and extracted three times with ethyl acetate 240ml water phase. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Analytical crystal solution with hydrogen chloride ether solution, filtered crystals with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,2-diphenylbutyric carbonitrile hydrochloride (2) as a white solid 7.0g, yield 76.8%, mp: 156.5-158. . . 1H-NmrgoomHzADCI3), δ (ppm): 7.35-7.42 (10H, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 (2H, m) , 2.25 (3H, s).
Preparation imidafenacin (I),
[0046] 4- (2-methyl-1-imidazolyl) -2,2_ diphenyl butyronitrile hydrochloride (2) (8.78g, 26mmol) in 70% concentrated sulfuric acid (25ml) was added to the reaction bottle, the reaction was stirred at 100 ° C, the end of the reaction was monitored by TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 7.lg, yield 85.5%, mp: 188.0-190. (TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
[0047] 1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2-),
3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H)
, 6.828 (s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H).
13C-NMR (CDC13,400MHz) δ: 12.17 (-CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
Example 41- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
[0051] The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), tetrabutylammonium bromide (TBAB) (0.5g) and K2C03 (3.6g), K0H ( 4.6g) were added sequentially 100mL three-necked flask, stirred and heated to 60 ° C reaction 4h.Cooling to room temperature, the reaction solution was filtered, and the filtrate was washed with saturated aqueous sodium bicarbonate, dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was dissolved with stirring parsing crystal give the product 4.5g, yield 78.1%, mp.79_80 ° C.
Preparation of [0052] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
[0053] The diphenyl acetonitrile (5.8g, 30mmol) and 50% aqueous KOH (15ml), dimethyl sulfoxide (DMSO) (100ml), tetrabutylammonium bromide (TBAB) (0.9g) in toluene 50ml was added to the reaction flask and stirred for 0.5h in the 40 ° C. Plus Λ 1- (2- bromoethyl) -2-methyl -1H- imidazole (4) (5.lg, 27mmol), was heated to 100 ° C, the reaction was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL of water and extracted three times with ethyl acetate 240ml water phase. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Analytical crystal solution with hydrogen chloride ether solution, filtered crystals with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,2-diphenylbutyric carbonitrile hydrochloride (2) as a white solid 6.7g, yield 73.4%, mp: 156.5-158 ° C. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.35-7.42 (IOH, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 ( 2H, m), 2.25 (3H, s).
The preparation imidafenacin (I),
4- (2-methyl-1-imidazolyl) -2,2_ diphenyl butyronitrile hydrochloride (2) (8.78g, 26mmol) in 70% concentrated sulfuric acid (25ml) was added to the reaction bottle, the reaction was stirred at 150 ° C at the end of the reaction was monitored TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 6.2g, yield 74.8%, mp: 188.0-190 (. TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
[0056] 1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2 -), 3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H)
, 6.828 (s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H).
13C-NMR (CDC13,400MHz) δ: 12.17 (-CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
Example 5
1- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), tetrabutylammonium bromide (TBAB) (0.5g) and K2CO3 (3.6g), K0H ( 4.6g) were added sequentially 100mL three-necked flask, stirred and heated to 20 ° C reaction 10h. Cooling to room temperature, the reaction solution was filtered, washed with a saturated aqueous sodium bicarbonate paint filtrate was dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was dissolved with stirring parsing crystal give the product 4.1g, yield 71.2%, mp.79-80. . .
Preparation of [0061] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
[0062] The diphenyl acetonitrile (5.8g, 30mmol) and 50% aqueous KOH (15ml), dimethyl sulfoxide (DMSO) (100mL), tetrabutylammonium bromide (TBAB) (0.9g) in toluene 50ml was added to the reaction flask and stirred at 20 ° C in Ih. Join
1- (2-bromoethyl) -2-methyl -1H- imidazole (4) (5.lg, 27mmol), was heated to 60 ° C, the reaction was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL of water and extracted three times with ethyl acetate 240ml water phase. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Solution of hydrogen chloride in ether solution with analytical crystal, crystals were filtered with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,
2-phenyl-butyronitrile hydrochloride (2) as a white solid 6.5g, yield 71.2%, mp: 156.5-158 ° C. 1H-NMR (400MHz, CDCl3), δ (ppm): 7.35-7.42 (IOH, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 ( 2H, m), 2.25 (3H, s).
[0063] Preparation of imidafenacin (I), [0064] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2) (8.78g, 26mmol ) and 70% of concentrated sulfuric acid (25ml) was added to the reaction flask, and stirred in at 50 ° C, the end of the reaction was monitored by TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 6.6g, yield 79.7%, mp: 188.0-190 (. TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
[0065] 1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2-),
3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H)
, 6.828 (s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H).
[0066] 13C-NMR (CDC13,400MHz) δ: 12.17 (-CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
[0067] Example 6
[0068] 1- (2-bromoethyl) -1H- -2_ methyl-imidazole (5) Preparation of
[0069] The 1,2_ dibromoethane (50ml), 2- methylimidazole (2.5g, 30.5mmol), benzyltriethylammonium chloride (TEBA) (0.35g) and Na2CO3 (2.8g), Na0H (3.3g) were added sequentially 100mL three-necked flask and stirred and heated to 40 ° C reaction 8h. Cooling to room temperature, the reaction solution was filtered, washed with a saturated aqueous sodium bicarbonate paint filtrate was dried over anhydrous sodium sulfate. Concentrated, added to a mixed solvent of isopropyl ether and ethyl acetate (3: 1) was dissolved with stirring parsing crystal give the product 4.4g, yield 76.4%, mp.79-80. . .
Preparation of [0070] 4- (2-methyl-1-imidazolyl) -2,2-diphenyl-butyronitrile hydrochloride (2)
[0071] The diphenyl acetonitrile (5.8g, 30mmol) and 50% aqueous KOH (15ml), dimethyl sulfoxide (DMSO) (100ml), benzyltriethylammonium chloride (TEBA) (0.66g) 50ml Toluene was added to the reaction flask and stirred 0.5h0 1- (2-bromoethyl) -2-methyl -1H- imidazole (4) at 40 ° C (5.lg, 27mmol), was heated to 50 ° C, the reaction mixture was stirred, TLC tracking and monitoring the reaction was complete, the mixture was poured into 100mL of water and extracted three times with ethyl acetate. The aqueous phase was 240ml. Washed three times with 300ml of water The organic phase was dried over anhydrous sodium sulfate, the organic phase was concentrated. Analytical crystal solution with hydrogen chloride ether solution, filtered crystals with a mixed solvent of isopropyl ether and recrystallized from ethyl acetate to give the condensation product of 4- (2-methyl-1-imidazolyl) -2,2-diphenylbutyric carbonitrile hydrochloride (2) as a white solid 6.8g, yield 74.6%, mp: 156.5-158. . . 1H-NmrgoomHzADCI3), δ (ppm): 7.35-7.42 (10H, m), 6.90 (1H, s), 6.77 (1H, s), 3.90-3.94 (2H, m), 2.75-2.79 (2H, m) , 2.25 (3H, s).
[0072] Preparation imidafenacin (I),
[0073] 4- (2-methyl-1-imidazolyl) -2,2_ diphenyl butyronitrile hydrochloride (2) (8.78g, 26mmol) in 70% concentrated sulfuric acid (25ml) was added to the reaction bottle, the reaction was stirred at 80 ° C, the end of the reaction was monitored by TLC tracking. The reaction solution was poured into 120ml of water, solid sodium carbonate was added to adjust PH to weakly alkaline, sufficiently stirred. With 180ml of dichloromethane and 35ml of ethanol mixed solvent was extracted three times, the organic phase was washed with water, dried over anhydrous sodium sulfate, the organic phase was concentrated. The residue was mixed with a solvent of ethyl acetate and recrystallized from ethanol to give 4- (2-methyl-1-imidazolyl) 2,2-diphenyl butyramide 6.Sg, yield 81.8%, mp: 188.0-190. (TC.1H-NMR and 13C-NMR data are as follows (see Figure 1-2 spectra):
[0074] 1H-NMR (CDC13,400ΜΗζ) δ: 2.209 (s, 3H, -CH3), 2.666-2.707 (t, 2H, -CH2-CH2-),
3.747-3.788 (t, 2H, -CH2-CH2 -), 5.341 (s, 1H, -NH -), 5.757 (s, 1H, -NH -), 6.699 (s, 1H, Ar-H)
, 6.828 (s, 1H, Ar-H), 7.287-7.390 (m, I OH, Ar-H). [0075] 13C-NMR (CDC13,400MHz) δ: 12.17 (_CH3), 41.00 (-CH2 -), 43.74 (-CH2-), 59.44 (quaternary carbon, coupled with strong electron-withdrawing group), 119.08 (-C = C -), 126.95 (aromatic carbon), 127.88 (aromatic carbon), 128.52 (aromatic carbon), 129.10 (aromatic carbon), 142.61 (= CN), 144.54 (-C = N), 176.21 (carbonyl carbon).
![]()
PATENT
CN 105399678
https://www.google.com/patents/CN105399678A?cl=en
CLIP
http://dmd.aspetjournals.org/content/35/9/1624/T3.expansion.html
TABLE 3
Chemical shifts of protons and carbons in 1H NMR and 13C NMR spectra of major (M-11b) and minor (M-11a) constituents of reference products obtained from imidafenacin![Graphic]()
Position of Proton
|
1H NMR Data (in D2O)
|
|
|
Major Constituent (M-11b)
|
Minor Constituent (M-11a)
|
| 1 |
2.18a (3Hb, sc) |
2.11a (3Hb, sc) |
| 2 |
2.82 (2H, m) |
2.79 (2H, m) |
| 3 |
3.45 (2H, m) |
3.41 (2H, m) |
| 5 |
5.26 (1H, s) |
5.43-5.47d (1H, d, J = 8.1e) |
| 6 |
5.33 (1H, s) |
5.43-5.47d (1H, d, J = 8.1e) |
| 8, 9, and 10 |
7.39-7.49 (10H, m) |
7.40-7.48 (10H, m) |
13
|
8.45 (1.3H, s)
|
8.45 (2H, s)
|
Position of Carbon
|
13C-NMR Data (in D2O)xc
|
|
|
Major Constituent (M-11b)
|
Minor Constituent (M-11a)
|
| 1 |
14.61a |
14.48a |
| 2 |
39.04 |
38.49 |
| 3 |
43.49 |
42.90 |
| 4 |
61.95-61.99f |
61.95-61.99f |
| 5 |
87.61 |
80.22 or 85.78f |
| 6 |
93.10 |
80.22 or 85.78f |
| 7 |
144.2-144.4f |
144.2-144.4f |
| 8, 9, and 10 |
130.7-131.8f |
130.7-131.8f |
| 11 |
170.8 |
169.5 |
| 12 |
181.9-182.2f |
181.9-182.2f |
13
|
173.8
|
173.8
|
![Fig. 1.]()
FIG. 1.
Chemical structures of [14C]imidafenacin and postulated metabolites, and their fragment ions. *, 14C labeled position; broken line, precursor and product ions obtained by collision-induced dissociation in LC/MS/MS.
| Cited Patent |
Filing date |
Publication date |
Applicant |
Title |
| CN101076521A * |
Dec 13, 2005 |
Nov 21, 2007 |
杏林制药株式会社 |
Process for producing muscarine receptor antagonist and intermediate therefor |
| CN102746235A * |
Jul 20, 2012 |
Oct 24, 2012 |
北京科莱博医药开发有限责任公司 |
Improved method for preparing imidafenacin |
| CN103275007A * |
May 27, 2013 |
Sep 4, 2013 |
朱雪琦 |
Pyrazole derivatives and preparation method thereof |
| US7351429 |
2008-04-01 |
Oral solid preparation |
| US2008004247 |
2008-01-03 |
Combinations of Statins with Bronchodilators |
| US2007270436 |
2007-11-22 |
NOVEL AMINO- AND IMINO-ALKYLPIPERAZINES |
| US2007219237 |
2007-09-20 |
Chromane Derivatives |
| US2007185129 |
2007-08-09 |
ACID ADDITION SALTS OF THIENOPYRANCARBOXAMIDE DERIVATIVES |
| US2007092566 |
2007-04-26 |
Oral sustained-release tablet |
| US2006188554 |
2006-08-24 |
Transdermal absorption preparation |
| EP0733621 |
2002-05-15 |
NOVEL IMIDAZOLE DERIVATIVE AND PROCESS FOR PRODUCING THE SAME |
| US6103747 |
2000-08-15 |
Imidazole derivatives and process for preparing the same |
| US5932607 |
1999-08-03 |
Imidazole derivatives and process for preparing the same |
| Patent ID |
Date |
Patent Title |
| US2015064232 |
2015-03-05 |
TRANSDERMAL ABSORPTION PREPARATION |
| US8729056 |
2014-05-20 |
Preventive and/or therapeutic agent of hand-foot syndrome |
| US8722133 |
2014-05-13 |
Method for production of orally rapidly disintegrating tablet comprising imidafenacin as active ingredient |
| US2013211352 |
2013-08-15 |
PERCUTANEOUSLY ABSORBED PREPARATION |
| US2013211353 |
2013-08-15 |
PERCUTANEOUS ABSORPTION TYPE FORMULATION |
| US8343544 |
2013-01-01 |
Oral sustained-release tablet |
| US2012289563 |
2012-11-15 |
COMBINATIONS OF IMIDAFENACIN AND SALIVARY STIMULANTS FOR THE TREATMENT OF OVERACTIVE BLADDER |
| US8247415 |
2012-08-21 |
Hydroxymethyl pyrrolidines as [beta]3 adrenergic receptor agonists |
| US8158152 |
2012-04-17 |
Lyophilization process and products obtained thereby |
| US8124633 |
2012-02-28 |
HYDROXYMETHYL ETHER HYDROISOINDOLINE TACHYKININ RECEPTOR ANTAGONISTS |
- Kobayashi F, Yageta Y, Segawa M, Matsuzawa S: Effects of imidafenacin (KRP-197/ONO-8025), a new anti-cholinergic agent, on muscarinic acetylcholine receptors. High affinities for M3 and M1 receptor subtypes and selectivity for urinary bladder over salivary gland. Arzneimittelforschung. 2007;57(2):92-100. [PubMed:17396619 ]
- Miyachi H, Kiyota H, Uchiki H, Segawa M: Synthesis and antimuscarinic activity of a series of 4-(1-Imidazolyl)-2,2-diphenylbutyramides: discovery of potent and subtype-selective antimuscarinic agents. Bioorg Med Chem. 1999 Jun;7(6):1151-61. [PubMed:10428387 ]
Reference
//////////イミダフェナシン , D06273, KRP-197, KRP 197, ONO-8025, ONO 8025, UNII:XJR8Y07LJO, Kyorin, Ono ,Imidafenacin, 170105-16-5, JAPAN 2015, Uritos® , Staybla®
Filed under:
JAPAN 2015,
Japan marketing,
Japan pipeline Tagged:
170105-16-5,
イミダフェナシン,
D06273,
Imidafenacin,
JAPAN 2015,
KRP-197,
KYORIN,
Ono,
ONO-8025,
Staybla®,
UNII:XJR8Y07LJO,
Uritos® ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()

 Japan
Japan





















 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..

 amcrasto@gmail.com
amcrasto@gmail.com


























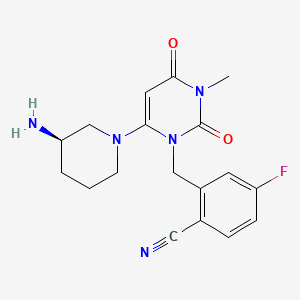

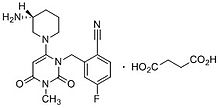
























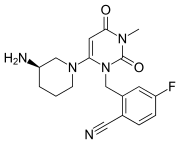































































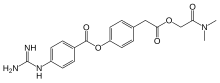
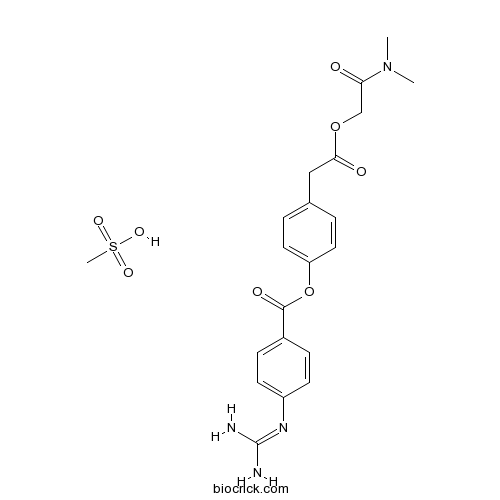

 CH4O3S : 494.52
CH4O3S : 494.52













.png)









Note: Compound name must be entered under “Substance Identification” and then “Names and Synonyms” selected to view synonyms.